On 20 January 2026, changes to the Australia New Zealand Food Standards Code were gazetted to permit food derived from a genetically modified (GM) Purple Tomato.
The GM Purple Tomato has been modified to naturally produce higher levels of blue pigments, called anthocyanins, as it ripens. This gives the tomato its purple skin and flesh. The modification also results in higher levels of chlorogenic acid, a naturally occurring compound found in many fruits and vegetables. These substances are commonly present in foods and are safe to eat.
FSANZ’s safety assessment found food derived from the GM Purple Tomato is as safe as food from conventional tomatoes already in the Australian and New Zealand food supply.
GM Purple Tomato and products containing it as an ingredient must be labelled as genetically modified, unless an exemption applies (see our GM labelling page).
The pre-market food safety assessment for this application was completed under the Health Canada-FSANZ Shared Assessment Process. It was the sixth GM food considered through this process.
Consultation and assessment
We released the public call for submissions in July 2025. Feedback from this consultation, along with our scientific assessment informed our final decision.
Approval
- A1333 Approval report (615KB)
- A1333 Support document (at Approval) (2.11MB)
Call for submissions
- A1333 Call for submissions (417KB)
- A1333 Supporting document (2.11MB)
- A1333 Application (11MB)
Submissions are available on the A1333 Consultation page.
Read our media release.
Administrative Assessment
- A1333 Administrative assessment report (126KB)
- A1333 Executive summary (40KB)
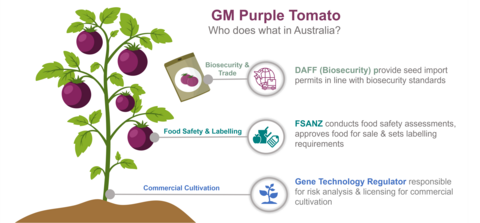
Cultivation and import
This application to FSANZ relates only to the use of the GM Purple Tomato as food in Australia and New Zealand.
On 6 January 2026, the Gene Technology Regulator (GTR) issued a license to Norfolk Healthy Produce's partner in Australia, All Aussie Avocados Ptd Ltd (trading as All Aussie Farmers), authorising the commercial release of the GM Purple Tomato throughout Australia. More information on this license is available on the OGTR website.
In New Zealand, commercial cultivation of the GM Purple Tomato would require separate regulatory assessment by the Environmental Protection Authority (EPA).
The importation of viable seeds into either country is subject to separate biosecurity and quarantine requirements. These are managed by the Department of Agriculture, Fisheries and Forestry (DAFF) in Australia and the Ministry for Primary Industries (MPI) in New Zealand.